David Escobar Laboratory
-
David Escobar Laboratory
- Principal Investigator
- Research
- Our Team
- Publications
- Careers

David Escobar, PhD
Assistant Staff
Director, Neural Dynamics and Modulation Lab
Email: [email protected]
Location: Cleveland Clinic Main Campus
Research
The goal of our research team is to advance the development of brain stimulation therapies that are tailored to the needs of each patient. This research leverages the fields of neurophysiology, data science, feedback (closed-loop) control, and dynamical systems to identify circuit-wide neural dynamics causally linked to the manifestation of brain conditions, and to develop closed-loop brain stimulation techniques that control these dynamics in real-time. Our group also develops technologies and methods to enable and advance research and innovation in the neuromodulation field. These technologies include portable electronic systems to deliver devices and multi-objective closed-loop stimulation therapies to objectively quantify motor performance in patients with movement disorders.
Biography
David Escobar, PhD, is Assistant Staff in the Department of Biomedical Engineering, Lerner Research Institute, and the Neurological Institute at Cleveland Clinic, where he directs the Neural Dynamics and Modulation Laboratory. He is an Assistant Professor at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. Dr. Escobar received his MS and PhD in aerospace engineering from the University of Minnesota in 2012 and 2015, respectively. His graduate research was directed at developing and validating robust feedback (closed-loop) control systems for uninhabited aerial vehicles and high-speed underwater vehicles that travel inside a gas bubble to increase their speed. In 2015, he joined the Department of Neurology at the University of Minnesota as a postdoctoral fellow. He conducted preclinical and clinical research on neurophysiology, the pathophysiology of Parkinson’s disease, neural signal processing, and closed-loop deep brain stimulation (DBS) technologies. In 2018, Dr. Escobar became a faculty in the Department of Neurology at the University of Minnesota, where he led research to accelerate the development of closed-loop DBS technologies for Parkinson’s disease. He joined the Cleveland Clinic in 2021 to establish a clinical research and technology development program aimed at
advancing personalized, computer-assisted DBS therapies for brain conditions by leveraging various engineering tools, including feedback control, optimization, data-driven mathematical modeling, signal processing, and data science.
Education & Professional Highlights
Education & Fellowships
- Postdoctoral Training
University of Minnesota Medical School, Department of Neurology, Minneapolis, MN
Focus areas: neurophysiology, movement disorders, deep brain stimulation (DBS), neural signal processing, data-driven mathematical modeling of brain circuits, and neural control engineering.
2015-2018 - PhD, Aerospace Engineering and Mechanics
University of Minnesota, Minneapolis, MN
Focus areas: data-driven mathematical modeling of nonlinear dynamical systems and robust feedback control.
2012-2015 - MS, Aerospace Engineering and Mechanics
University of Minnesota, Minneapolis, MN
Focus areas: design and validation of robust feedback control systems for autonomous aerial and underwater vehicles.
2010-2012 - Engineering Degree, Mechatronics Engineering
National University of Colombia, Bogota, Colombia
Focus areas: control systems, signal processing, robotics, computer science.
2003-2008
Appointments
- Assistant Staff
Department of Biomedical Engineering, Lerner Research Institute
Center for Neurological Restoration, Neurological Institute
Cleveland Clinic
2021-present - Assistant Professor
Department of Biomedical Engineering
Cleveland Clinic Lerner College of Medicine of Case Western Reserve University
2022-present - Assistant Professor
Department of Neuroscience, School of Biomedical Sciences
Kent State University
2023-present
Research
Overview
Our laboratory is dedicated to advancing neuromodulation therapies for brain conditions, translating research into medical technology, training students and mentees in neuroengineering, and partnering with clinicians, scientists, and engineers to bring neuromodulation advancements to patients.
Research: Our team conducts clinical and preclinical research to increase the effectiveness of brain stimulation therapies. This research aims to address the variability in outcomes of current therapies within and among patients. We integrate neurophysiology, feedback control engineering, signal processing, data science, and data-driven mathematical modeling to characterize neural circuit dynamics underlying brain dysfunction, and to develop personalized brain stimulation approaches that control these neural dynamics in real-time.
Technology development and innovation: Part of our investigative efforts surround developing new technologies to advance our research, including the portable electronic systems to deliver multi-objective closed-loop brain stimulation and the devices themselves, to objectively quantify motor performance in patients with Parkinson's disease. Our technology innovation work is also dedicated to translating our research into neuromodulation devices and practical therapies.
Education and training: We are committed to education to expand the network of scientists, engineers, and clinicians working to improve the lifestyle of people suffering from brain conditions. We provide multidisciplinary training and career mentorship to undergraduate and graduate students, postdoctoral fellows, visiting researchers, medical trainees, and volunteers interested in a career in engineering, science, and medical technology innovation.
Collaboration and outreach: We work closely and collaborate with a multidisciplinary team of clinicians, scientists, and engineers across the Cleveland Clinic Neurological Institute, Imaging Institute, and Lerner Research Institute to develop and test comprehensive neuromodulation treatments for distinct brain conditions, including Parkinson's disease, essential tremor, and stroke.
Projects
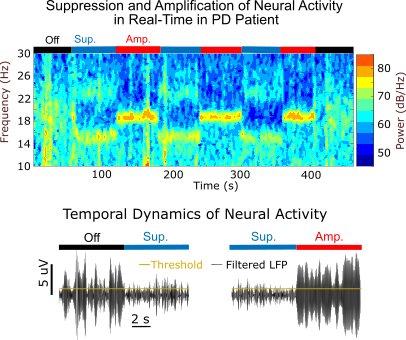
Identifying Circuit Dynamics Underlying Motor Dysfunction in Parkinson's Disease Using Data-Driven Modeling and Real-Time Neural ControlThe goals of this project are to identify circuit-wide neural dynamics linked to the manifestation of Parkinson’s disease (PD) motor signs and to develop new deep brain stimulation (DBS) technology to control these neural dynamics in real-time.
Current theories propose that elevated 8-35 Hz oscillations, synchronized throughout the basal ganglia-thalamocortical (BGTC) circuit, are associated with rigidity and bradykinesia in Parkinson's disease. The circuit-wide dynamics underlying the generation of these oscillations and their causal relationship with specific motor signs, however, remains unclear. Clarifying this relationship is critical to advancing our understanding of PD pathophysiology and developing deep brain stimulation (DBS) therapies optimized to control neural processes underlying motor dysfunction in PD.
We are leveraging a new neural control approach we developed to suppress or amplify frequency-specific neural activity in real-time to characterize how controlled changes in 8-35 Hz oscillations in the internal segment of the globus pallidus (GPi) or the subthalamic nucleus (STN) influence the severity of motor signs in PD patients. This technique, referred to as evoked-interference closed-loop DBS (eiDBS), delivers stimulation with precise magnitude and timing to evoke neural responses that modulate spontaneous activity in a targeted neuronal population.
The data from this project will help clarify the causal role of GPi and STN 8-35 Hz oscillations in the manifestation of PD motor signs, and advance the development of closed-loop DBS technology that precisely controls oscillatory dynamics linked to PD.
Click here to read a story about this project on the ConsultQD website.
Optimizing Electrode Placement and Programming for Deep Brain Stimulation in Parkinson’s Disease
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) or internal segment of the globus pallidus (GPi) is the standard treatment when Parkinson’s disease (PD) medications no longer provide adequate motor control. DBS systems deliver isochronal, high-frequency (~130 Hz) electrical pulses to the STN to alleviate motor impairment. The most critical factor for DBS to be effective with minimal side effects is to implant the DBS leads in the sensorimotor region of the STN or GPi. Errors in the targeting location can decrease the therapeutic effectiveness and increase the likelihood of side effects due to the spread of electric currents into regions not targeted for stimulation, including non-motor areas of the STN and the internal capsule. The most common side effects include paresthesia (numbness, tingling, electrical sensations); muscle tightening, pulling, or cramping in the face, neck, trunk, and limbs; and dysarthria (slurring speech or trouble speaking). Several factors can contribute to errors in the surgical implantation of DBS leads, including brain shifts due to leakage of cerebrospinal fluid or air buildup, subjective measures of the patient’s clinical state in the operating room (OR), and the experience of the clinicians performing the electrophysiological mapping. Newer DBS leads offer directionally oriented electrode contacts that can “rescue” misplaced leads by directing current to specific areas to maximize the activation of neuronal elements linked to therapeutic benefit and minimize the activation of tissue associated with side effects. However, the programming of these directional leads is based on suboptimal and time-consuming behavioral assessments. Robust and efficient brain mapping and DBS programming methodologies based on patient-specific physiological data are needed to automate and optimize therapy outcomes and reduce healthcare costs. We are addressing this unmet need in our research and technology development program.
Objectively Quantifying Rigidity to Optimize Therapies for Parkinson’s Disease
Rigidity affects the majority of Parkinson’s disease (PD) patients and is characterized by stiff and inflexible muscles that prevent relaxation. Objectively quantifying rigidity in PD is vital to evaluating and advancing therapies such as deep brain stimulation (DBS). In both clinical and research settings, the Unified Parkinson’s Disease Rating Scale (UPDRS-III) is the most widely used approach for assessing rigidity. To obtain the rigidity sub-score, the examiner manipulates major joints and assigns a 0-4 rating (increments of 1) based on the rigidity’s severity. The UPDRS-III scale has low sensitivity to quantify mild severity or small changes in motor signs due to its coarse rating and dependence on human perception, and can exhibit limited inter-rated reliability. We are developing robotic systems and assessment methodologies to objectively quantify rigidity of multiple joints in humans. Our robotic systems are capable of generating torque perturbations while the subject moves the arm freely to separate the effect of these perturbations from the torque generated by the subject when tremor, dyskinesia, or voluntary movement is present. Our ultimate goal is to use our robotic systems and assessment methodologies to characterize the physiological mechanisms underlying rigidity and objectively assess the effectiveness of DBS therapies.
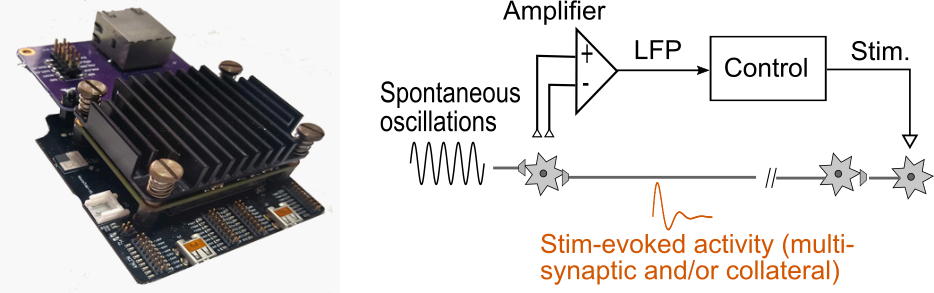
Electrical Stimulation Technologies and Feedback Control Strategies to Modulate Brain Circuitry in Real-Time
The goal of this project is to conceive and test feedback control technologies to precisely modulate brain activity in real-time. We are developing hardware and software tools to validate and rapidly prototype signal processing and feedback control algorithms for neural modulation. These technologies will enable us to characterize the role of neural activity in the manifestation of brain conditions and to advance the development of personalized neuromodulation therapies.
Media
NeuroNews International, ‘Proof-of-concept study indicates utility of evoked-interference DBS in Parkinson’s disease,’ 12/2022. Click this link to read the article.
Our Team
Selected Publications
Careers
We are always looking for talented students, postdocs, engineers, clinicians, and researchers to join our team. If you are interested in working with us and have expertise in any of the topics listed below, please contact Dr. David Escobar at [email protected].
- Signal processing
- Dynamical systems and feedback control engineering
- Data-driven mathematical modeling of dynamical systems
- Computer programming using Python, C++, MATLAB/Simulink
- Data science, machine learning, and deep learning
- Real-time computing
- Finite element modeling and electromagnetism
- Robotics
- Neuromodulation, deep brain stimulation
- Movement disorders
- Electrophysiology (EEG, iEEG, ECoG, MEG, and EMG)
- Computational neuroscience
Training at Lerner Research Institute
Our education and training programs offer hands-on experience at one of the nationʼs top hospitals. Travel, publish in high impact journals and collaborate with investigators to solve real-world biomedical research questions.
Learn More